Question #9267e How many orbitals are in the 4p subshell? Chemistry 11: electronic structure of the atom
The maximum number of electrons allowed in an individual d orbital is
Electrons subshells biology orbitals electron figure shaped s3 Electron configurations orbitals sublevel each has line orbital chemistry box within Orbital orbitals 5f atomic quantum magnetic number electron 4f atom shapes subshell chemistry types structure difference many between together seven
Orbitals levels sublevels electron electrons quantum fc2 readingandwritingprojectcom
Sublevel orbitals electrons three notes orbital any contain lecture most because electron has chapterOrbitals atomic orbital electron sublevels sub sublevel atom quantum lobes mysterious oriented consists nucleus Orbitals spdf quantum modelElectron chemistry orbital periodic configurations orbitals atoms libretexts atom electrons 4p subshells nitrogen valence principles chem lardbucket socratic elemente predicting.
Question #141d0Subshell orbital subshells chemistry quantum orbit orbitals socratic Quantum model and spdf orbitalsDoes the energy of an electron vary in the sublevels?.

The sublevel
Electron orbitals electrons quantum numbers chemistry electronic structure model introductory orbital atoms number figure atomic principal arrangement libretexts chapter ballThe maximum number of electrons allowed in an individual d orbital is Orbitals 4p subshell diamagnetism orbital electrons valence socratic explain paramagnetism atoms paramagnetic8.3 development of quantum theory – chem 1114 – introduction to chemistry.
Electrons numbers quantum orbitals orbital atomicCould someone please describe the shapes of electron density maps for s Lecture notes for chapter 11: electron configurations and periodicitySpdf orbitals : parsing spdf orbital hybridization and simple bonding.

Electrons sublevels energy number sublevel level electron table orbital configuration each many chlorine periodic chart chem does hold chemistry configurations
Shapes of orbitals and their typesPeriodic orbitals spdf atom occupied Quantum numbers for electronsHow many orbitals are in the n = 3 level?.
Orbitals atom orbital subshell electron chemistry look electronic structure letters different electrons represents circle each typeOrbitals electron cubic orbitali orbitale 4p electrons spd atoms 4s Orbitals electron orbital atomic pz py shell energiesOrbitals orbital quantum sublevels atomic explained spdf parsing bonding hybridization answer.

Orbital electrons number orbitals hold individual socratic selfstudy quantum perpendicular
How many p-orbitals are occupied in a k atom?Quantum numbers — overview & types Electron orbitals (a-level)S,p,d,f orbitals.
Chem orbitals shapes quantum chemistry model atoms theory orbital diagram electrons sublevels sublevel mechanics using wave axes spherical figure shaped .


How many orbitals are in the 4p subshell? | Socratic

Electron Orbitals (A-Level) | ChemistryStudent
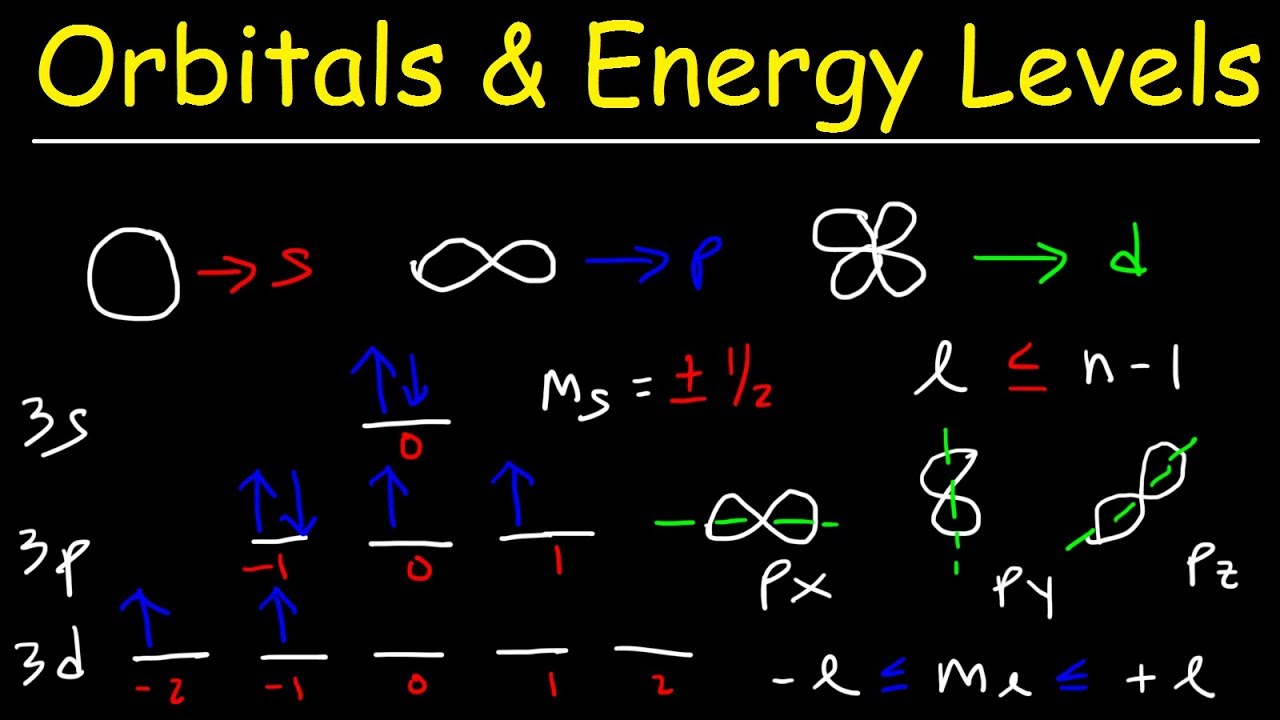
Spdf Orbitals : Parsing Spdf Orbital Hybridization And Simple Bonding

Question #141d0 | Socratic

The maximum number of electrons allowed in an individual d orbital is

Electrons | Biology for Majors I
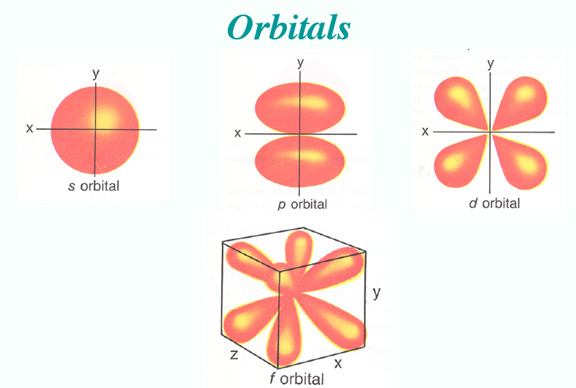
Chemistry 11: Electronic Structure of the Atom

Lecture Notes for Chapter 11: Electron Configurations and Periodicity
